
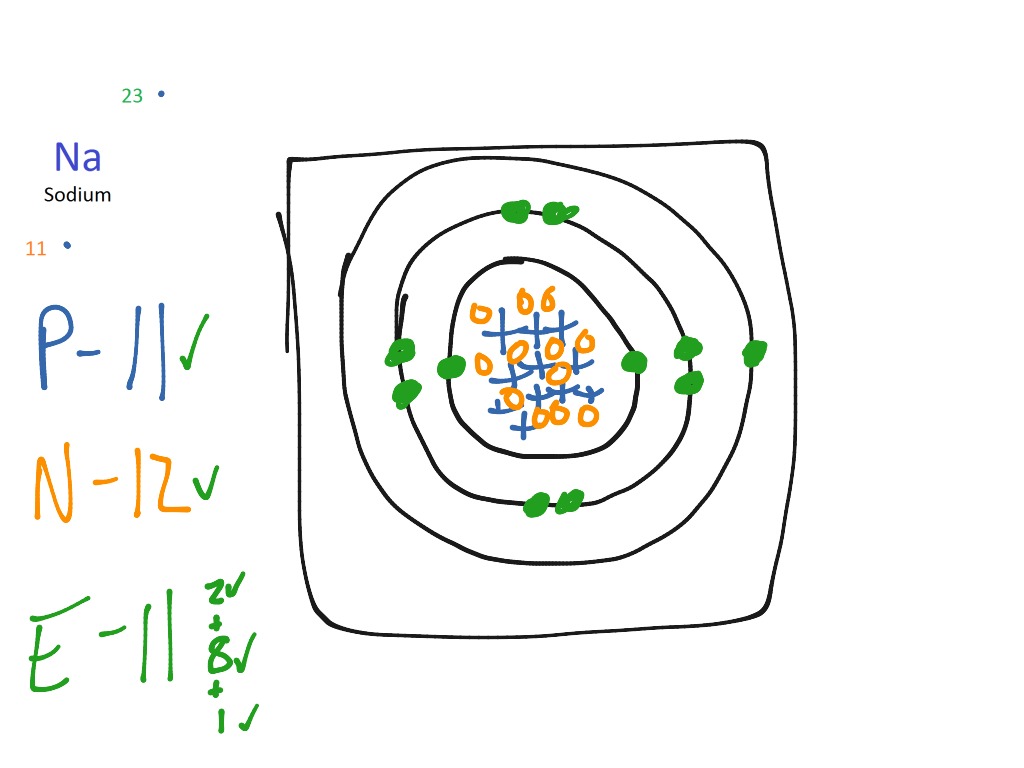
Sodium Bohr model
That is, sodium is a cation element. Na - e - → Na +. The electron configuration of sodium ion (Na +) is 1s 2 2s 2 2p 6. This electron configuration shows that sodium ion (Na +) has acquired the electron configuration of neon (Ne) and it achieves an octave full stable electron configuration.

Sodium Bohr Model — Diagram, Steps To Draw Techiescientist
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

Atom illustration, Bohr model Sodium Atom Chemistry Rutherford model
Steps to draw the Bohr Model of Sodium atom 1. Find the number of protons, electrons, and neutrons in the Sodium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom

Figure \ Electron Shell Configuration Of Sodium 1200x1200 PNG
The main points of Bohr's model are as follows: 1. Electrons move in fixed orbits and not anywhere in between. 2. Each orbit has a fixed size and energy. The energy of the orbit is related to.

Sodium Facts & Bohr Model YouTube
Physical & Theoretical Chemistry Supplemental Modules (Physical and Theoretical Chemistry) Electronic Structure of Atoms and Molecules
Bohr Model Of Sodium
With sodium, however, we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum, at about 589 nm. Figure \(\PageIndex{1}\): The Emission of Light by Hydrogen Atoms. (a) A sample of excited hydrogen atoms emits a characteristic red light.. Bohr's model required only one assumption:.

Bohr Diagram Of Sodium Atom model, Atom diagram, Atom model project
The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons. As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr model represents these energy levels as rings. We can tell that the two electrons in the model above are at the same energy.
.PNG)
Bohr Models and Lewis Dot Diagrams Presentation Chemistry
Niels Bohr adapted Ernest Rutherford's nuclear model. Bohr did calculations that led him to suggest that electrons orbit the nucleus in shells. The shells are at certain distances from the nucleus.
Sodium (Na) AMERICAN ELEMENTS
atom On the Web: Space.com - The Bohr model: The famous but flawed depiction of an atom (Jan. 08, 2024) See all related content → Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.

Atom Sodium Model Stock Illustration Download Image Now iStock
Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.
Sodium (Na) AMERICAN ELEMENTS
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV

Bohr Diagram For Calcium Wiring Diagram
It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10 -19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations.
Bohr Model of Sodium Science ShowMe
or. 1 λ = k hc( 1 n2 1 − 1 n2 2) The lowest few energy levels are shown in Figure 3.2.1. One of the fundamental laws of physics is that matter is most stable with the lowest possible energy. Thus, the electron in a hydrogen atom usually moves in the n = 1 orbit, the orbit in which it has the lowest energy.
How To Draw Bohr Models For Ions
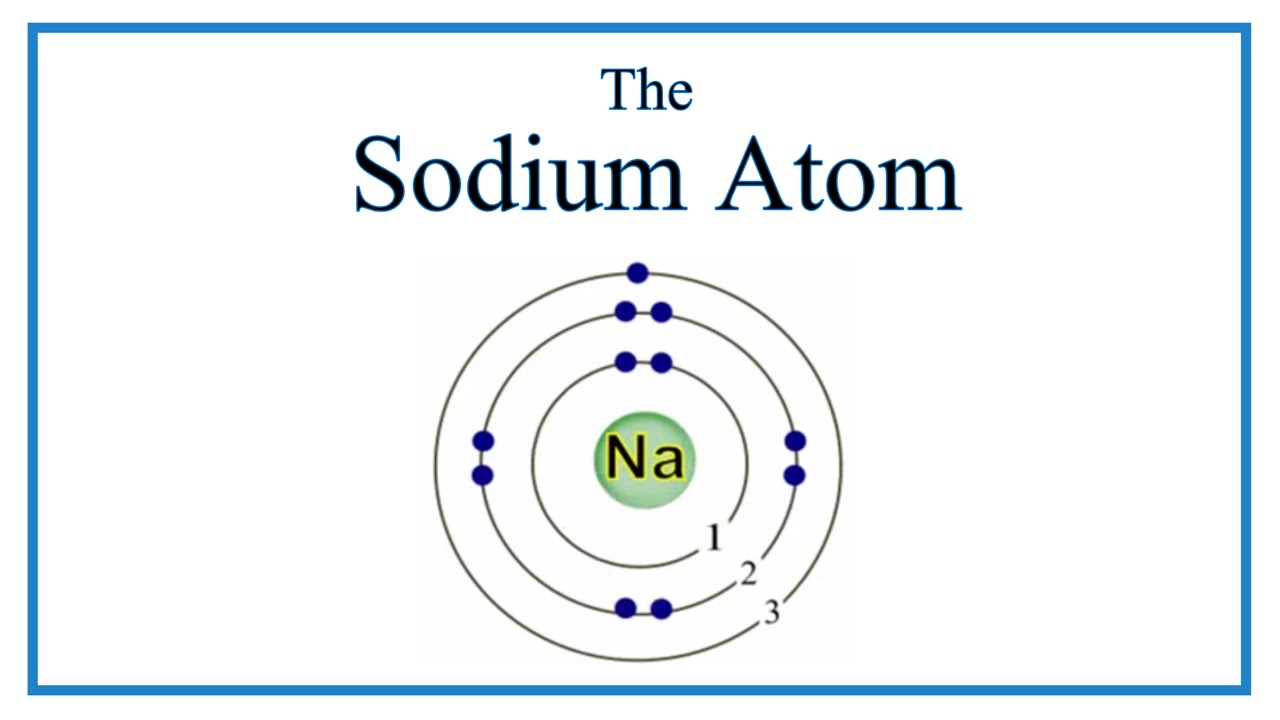
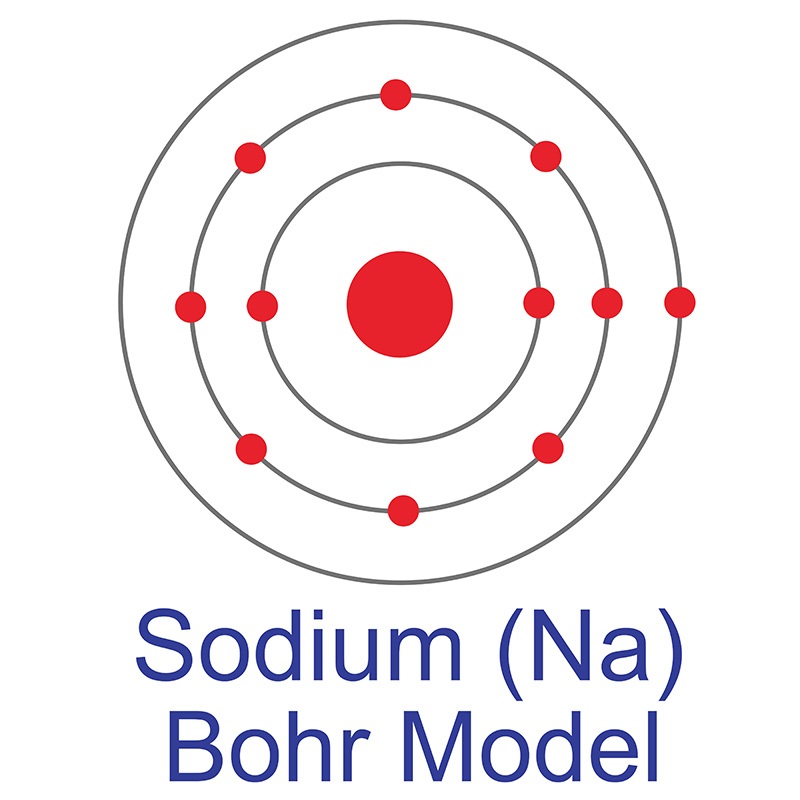
The Bohr Model of Sodium. Let's look at the Bohr model of sodium, which has 11 electrons. Image Courtesy of Wikimedia. The atomic number of sodium is 11, which indicates that there are both 11 protons and electrons. This is why there are 11 electrons represented in the above diagram.

ScIU Conversations in Science at Indiana University
Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which is closer to nucleus has less energy Postulates of Bohr's Model of an Atom
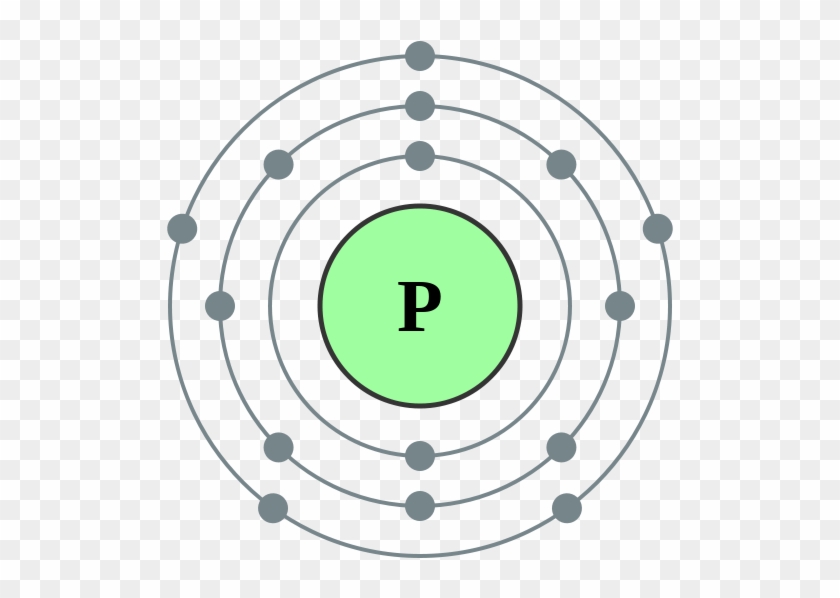
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
The Bohr model of sodium contains a nucleus having 11 protons and 12 neutrons in the center, and around this nucleus, there are three electron shells containing 11 electrons. Atomic Structure of the Sodium Atom (Na) Watch on Contents Steps #1 Write protons, neutrons, and electrons of sodium atom #2 Draw nucleus of sodium atom